Understand, position & prepare
Getting things right from the start is crucial when launching. The margins for error are relatively small and the cost of missing information can be very high.
A solid starting point for every launch team should be to measure the shape it is in before creating the timeline to market entry. Being conscious about what you know – and more importantly – what you don’t know is not something to review lightly. Asking yourself key strategic questions such as “What and how to prioritize in this launch journey?” or “Do we have a precise definition of the market opportunity, the market size, and the competitive landscape?” allows the identification of any gaps in launch plans early on.
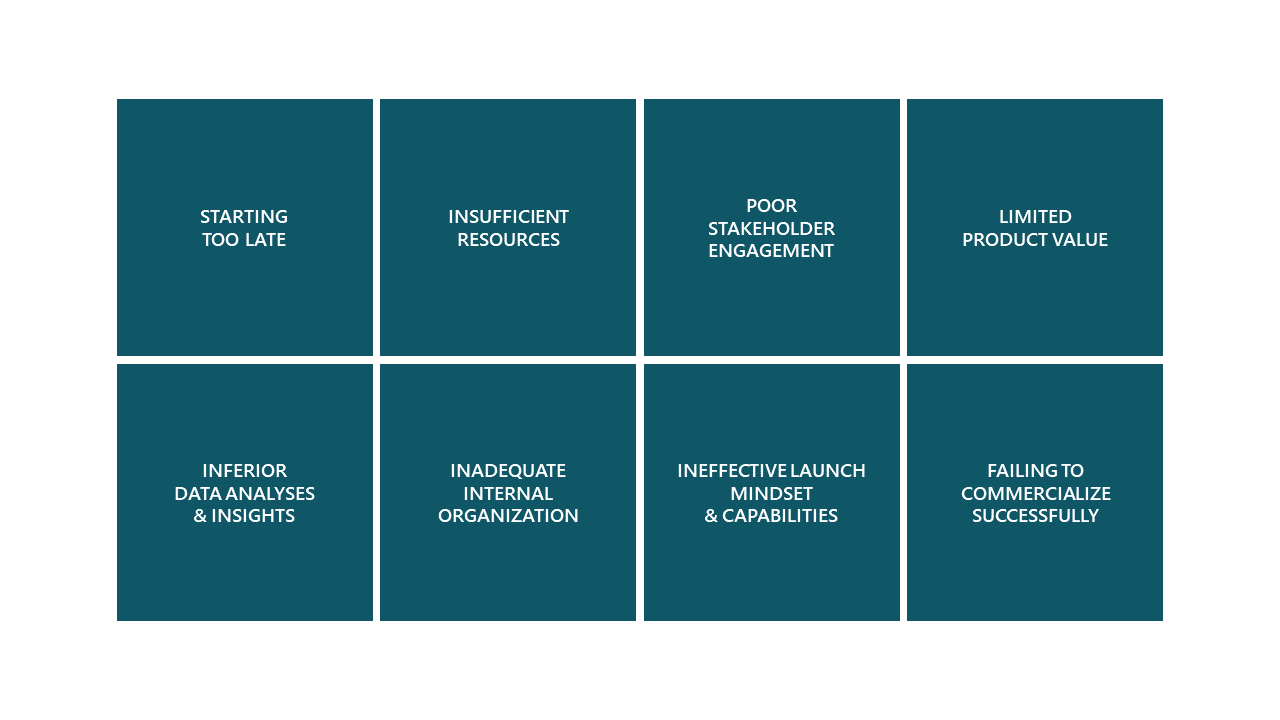
There exist several common pitfalls when preparing for launch (figure 3). Investigating and/or exploring each of these with a cross-functional team can identify potential challenges, but also reveal equally valuable opportunities in your road to meeting launch expectations and forecasts.
 The true strength of a solid launch preparation, however, is to continuously improve the team’s understanding of the market dynamics. Most teams are familiar with initiating a periodic tracking or market sensing in order to keep up-to-date with any changes in their playing field, although this is most often strongly focused on – and limited to - brand performance.
The true strength of a solid launch preparation, however, is to continuously improve the team’s understanding of the market dynamics. Most teams are familiar with initiating a periodic tracking or market sensing in order to keep up-to-date with any changes in their playing field, although this is most often strongly focused on – and limited to - brand performance.
Where classic market sensing in launch (e.g., ATU studies) solely focuses on the product itself, the decisions of prescribers are influenced by many more factors.
How are the interactions by the pharma company’s representatives (both commercial and medical) impacting this decision? Is the product endorsed by key thought leaders in that space? Are there any barriers for prescribing or having access to the product? Are there specific patient associations or patient profiles that request the product themselves? How valuable are the medical education meetings and congresses organized by the pharma company to the HCPs?
Integrating all these aspects of your market in a holistic view while tracking market dynamics will provide launch teams with much more granular insights and give them the ability to link insights from these various domains to a solid launch strategy.
Benchmarking: knowing where you are at any moment
Gaining all these insights in various areas of your business reality and target market is crucial, but capturing them is only the beginning. Drawing the correct conclusions from this continuous stream of information is an important next step.
Most launch teams receive insights from market research and/or other info sources and are challenged with finding a correct way of interpreting them. Having a comparator to benchmark against is definitely helpful. But what if such a comparator doesn’t exist in your market? For example, in situations in which no direct (or strategic) competition is identified or markets where you are the first to enter. How do you evaluate your performance then?
Industry benchmarks are a solid tool for tracking if your launch is on its way to being successful, based on a variety of brands who’ve done the trick before you. Not only do they provide a single view on your current performance, but they also visualize the uptake that is needed in certain metrics or KPIs to make an impact in the market.
A common example of benchmarks are case studies on launch successes in the disease or therapeutic area of your interest. By learning from what those before you did well or were challenged with, you gain insights into which barriers to overcome and which opportunities to exploit. But even if such (successful) cases don’t exist for your particular situation, you can still refer to wider industry benchmarks available.
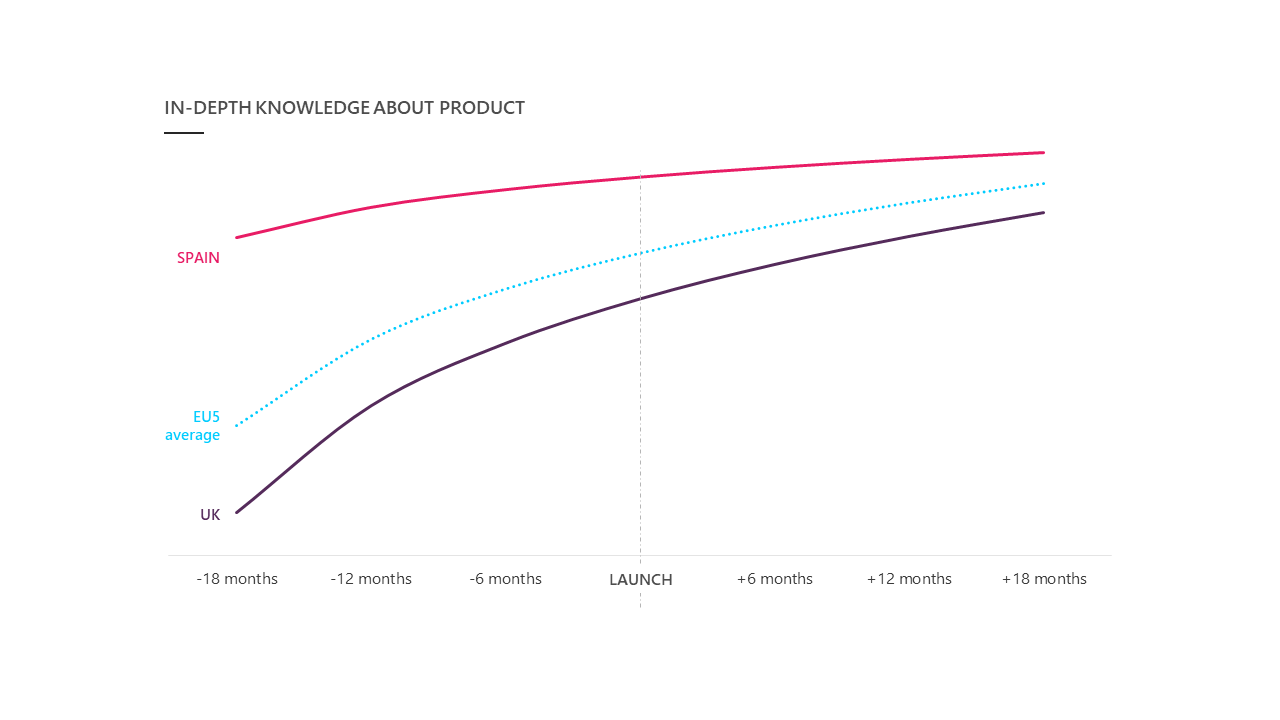
The launch-aligned benchmarks for in-depth product knowledge across EU5 markets (figure 4) indicates that launching successfully is strongly dependent on context. In the UK, we see that the knowledge of HCPs on the new product requires a steep uptake and continues to grow even after launch. In Spain, knowledge levels should be (or already are) built well upfront as shown by the higher starting positions of the curves at 18 months before launch. However, the total uptake of knowledge levels in this country is significantly lower than in the EU5 average.
After launch in Spain, knowledge levels remain status quo within successful launches.
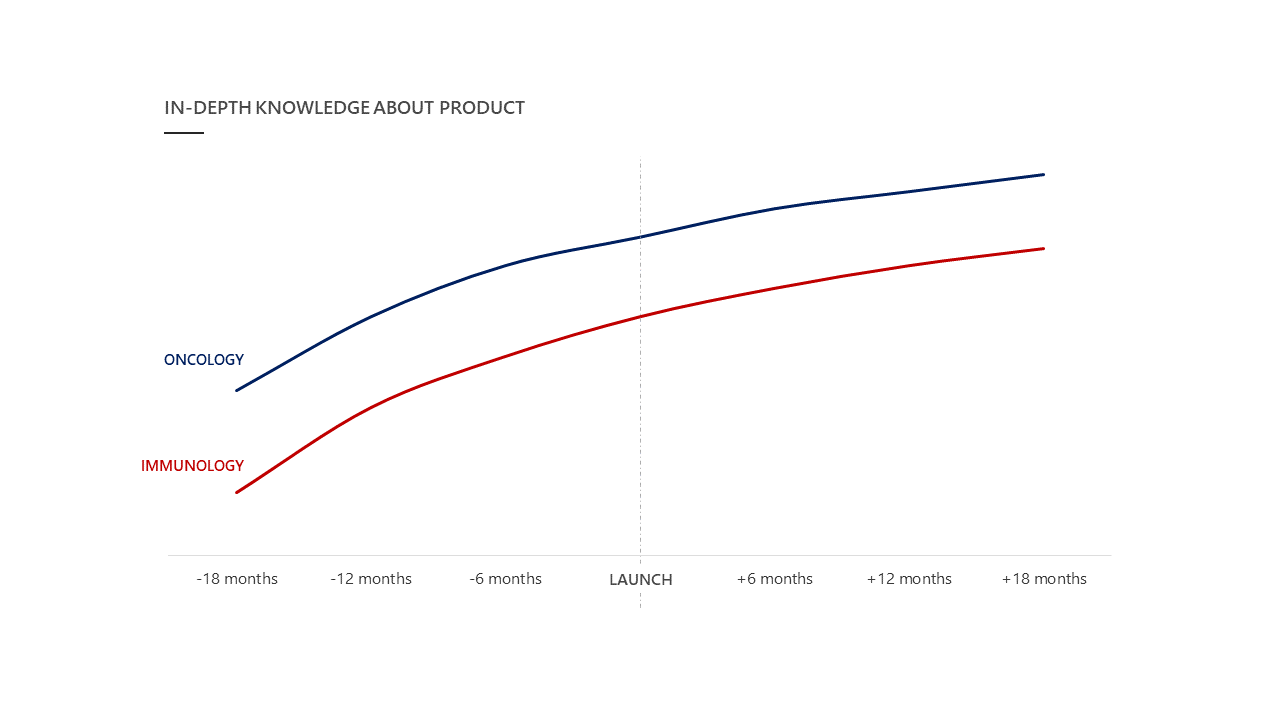
Another approach is comparing to successful historic launches within your therapeutic area. Considering in-depth product knowledge once again (figure 5) demonstrates how in oncology, expectations about HCPs’ knowledge about new products are significantly higher than in immunology. Within the latter therapeutic area, the strongest uptake in product knowledge is required to be successful.
THE BALANCING ACT OF PRODUCT LAUNCHING
It has become clear that being successful in launching a product is a huge endeavor.
The stakes are not only big in terms of the R&D expenses that are sunk already before any returns happen, the amplified competitiveness in the markets over the years has also made it increasingly difficult for new products (and their promoting companies) to stand out in the market. A solid preparation in which various elements are considered well upfront is key, allowing the identification of potential gaps but also opportunities during your launch journey.
In addition, tracking key metrics and consistently engaging in market sensing during a launch contributes to understanding your market, identifying barriers, drivers, and assessing the competitive landscape. Moreover, by investigating several domains on top of a single product, focus will allow for deeper insights and the interlinking of your planned activities.
But data and insights themselves will be difficult to interpret if there are no comparators in place. Historic cases of successful launches can prove valuable, but demand for such cases typically outstrips supply. Launch-aligned benchmark curves and scores can offer a solid alternative and can provide analytical proof of what is crucial to focus and work on in your specific target market and therapeutic area.
SOURCES & REFERENCES
FIGURE 1: Datamonitor Healthcare | Patient-based forecast (2017 – 2030) – EU5, US & JPN
FIGURE 2: Trilations | Competitive dynamics research
FIGURE 3: Trilations | Keynote at EyeForPharma Japan (2020), “The Most Common Pitfalls in Pharmaceutical Product Launch (and tips and tricks on how to avoid them)”
FIGURE 4: Trilations | Competitive dynamics research
FIGURE 5: Trilations | Competitive dynamics research
More players in the target markets for new compounds also implies less differentiation between the most preferred treatments within a disease area. Whereas in 2018 the perceived differentiation between the top five treatments was still 27%, this has declined to only 19% in 2023 (figure 2).
A logical result of the situation described above is that it has become increasingly difficult for new compounds to successfully differentiate and meet forecasts. From the drug launches that lagged their forecast in year one, 78% continue to do so in year two and 70% don’t even meet forecasts in year three. Once a product fails in meeting the expectations built in the market, it becomes extremely tough to turn the tide.


 The true strength of a solid launch preparation, however, is to continuously improve the team’s understanding of the market dynamics. Most teams are familiar with initiating a periodic tracking or market sensing in order to keep up-to-date with any changes in their playing field, although this is most often strongly focused on – and limited to - brand performance.
The true strength of a solid launch preparation, however, is to continuously improve the team’s understanding of the market dynamics. Most teams are familiar with initiating a periodic tracking or market sensing in order to keep up-to-date with any changes in their playing field, although this is most often strongly focused on – and limited to - brand performance.


